Background: Treatment of chronic lymphocytic leukemia (CLL) has been revolutionized by usage of targeted therapies against the B-cell receptor (BCR) signaling cascade, specifically Bruton's Tyrosine Kinase (BTK). However, resistance to BTK inhibition occurs through acquisition of various mutations in BTK or its immediate downstream signaling partner, PLCγ2. Clinical outcomes for these patients are poor, emphasizing the need for alternative therapeutics. In the presence of these mutations, BCR signaling remains intact, suggesting that targeting molecules downstream of BTK may be effective. Protein kinase C-β (PKCβ) is a downstream component of the BCR pathway that has been shown to be over-expressed in CLL. Moreover, PKCβ is essential to the development of CLL in the Eμ-TCL1 mouse model and its expression in stromal cells is required for the survival of leukemic B-cells (Holler et al. 2009). This suggests that signaling through PKCβ, in both CLL cells and cells in the microenvironment, is essential to promote leukemogenesis. MS-553 is a potent, ATP competitive, reversible inhibitor of multiple PKC isoforms including PKCβ, with the potential to overcome BTKi mediated resistance.
Methods: Primary CLL cells were isolated by negative selection and treated with up to 5 μM MS-553. Cell viability was assessed via Annexin V/PI with and without HS-5 stromal cell co-culture. BCR and canonical WNT signaling changes were evaluated by change in target protein phosphorylation or total protein by immunoblot following drugging. Changes in downstream NFκB and WNT signaling were interrogated by RT-qPCR. Using genetically modified TMD8 cells that express only C481S or T474I mutated BTK, we tested MS-553's effect on cell-viability and BCR signaling. In vivo survival studies were performed using the Eμ-MTCP1 (Walker et al. 2021) adoptive transfer mouse model, an aggressive model of CLL, with subsequent randomization into vehicle or MS-553 treatment arms.
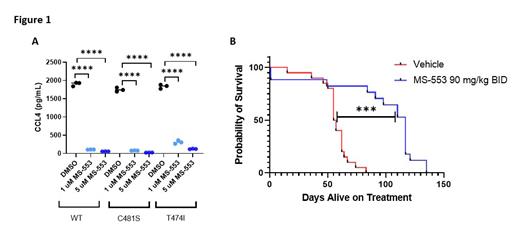
Results: Following 72 hours of drugging, 5 μM MS-553 displayed modest cytotoxicity against primary CLL cells both alone (50%, p<0.0001, n=8) and in the presence of HS-5 stromal cells (25%, p=0.0008, n=8). In primary CLL cells harboring the C481S BTK mutation, we observed retained cytotoxicity following 72 hours of drugging (29%, p=0.00029, n=6). At 24 hours, 5 μM MS-553 inhibited downstream BCR and WNT signaling in primary CLL cells, demonstrated by reduced phosphorylation of PKC-β (43%, p=0.0024, n=15) and several of its downstream targets including pGSK3β (45%, p<0.0001, n=15), pIκBα (66%, p<0.0001, n=15), and pERK (17%, p=0.0301, n=15), and reduced total protein of β-Catenin (82%, p<0.0001, n=9), Cyclin D2 (71%, p=0.0011, n=9), and cMyc (85%, p<0.0001, n=9) compared to vehicle treated, stimulated samples. In both treatment naïve and C481S patient samples, mRNA expression of NFκB and WNT pathway targets BCL-2, OCT-2, BFL-1, MYC, CD40, Cyclin D1, and Cyclin D2 were all decreased after 48 hours of treatment with 1 or 5 μM MS-553. MS-553 also showed efficacy in the presence of BTK mutations: viability in TMD8 cells was reduced following 72-hour drugging with MS-553 regardless of BTK mutational status (WT: 22%, p=0.0006, n=3; C481S BTK: 18%, p=0.0032, n=3; T474I BTK: 19%, p=0.0015, n=3). Inflammatory cytokine production is inhibited by MS-553 through its ability to decrease CCL3 and CCL4 cytokine expression in WT and mutant BTK-harboring TMD8 cells (p<0.0001, n=3) (Figure 1A). To validate inhibition of BCR signaling in the C481S BTK setting, we treated primary CLL cells harboring this mutation and observed a reduction in both CCL3 and CCL4 production (61%, p=0.001229, n=5). As proof of concept, we examined MS-553's efficacy in vivo in the Eμ-MTCP1 mouse engraftment model, and found a significant reduction in disease progression and increase in survival (median 117 days) when compared to vehicle control (median 56 days, p=0.0003) (Figure 1B).
Conclusion: Our data demonstrate the efficacy of the PKCβ inhibitor MS-553 in preclinical models of CLL including that with BTK mutations conferring BTKi resistance. These findings support continued preclinical and clinical investigation of MS-553 in CLL, and the phase 1 trial of this agent is currently underway (NCT03492125) (Blachly et al. 2022).
Disclosures
Rogers:Janssen: Consultancy; AstraZeneca: Consultancy; Beigene: Consultancy; Pharmacyclics: Consultancy; Novartis: Research Funding; Loxo@Lilly: Consultancy; AbbVie: Consultancy, Research Funding; Genentech: Consultancy, Research Funding. Blachly:AbbVie: Consultancy; Leukemia Diagnostic Device: Patents & Royalties: Being prosecuted; Epigenetic classification of leukemia: Patents & Royalties: PCT conversion filed; AstraZeneca: Consultancy; Astellas: Consultancy. Kittai:Abbive: Consultancy; AstraZeneca: Consultancy, Research Funding; BeiGene: Consultancy, Research Funding, Speakers Bureau; Eli Lilly: Consultancy; Janssen: Consultancy; KITE: Consultancy; BMS: Consultancy. Bhat:Aptitude Health: Honoraria; Abbvie: Consultancy; AstraZeneca: Consultancy, Research Funding. Byrd:Orbimed: Consultancy, Research Funding; OSU Drug Devel. Inst.: Consultancy; Eilean Therapeutics: Consultancy, Current equity holder in private company, Membership on an entity's Board of Directors or advisory committees, Research Funding; Vincerx: Current equity holder in publicly-traded company, Membership on an entity's Board of Directors or advisory committees; Kurome: Current equity holder in publicly-traded company, Membership on an entity's Board of Directors or advisory committees; Newave: Membership on an entity's Board of Directors or advisory committees, Research Funding; Orange Grove Bio: Membership on an entity's Board of Directors or advisory committees; American Cancer: Membership on an entity's Board of Directors or advisory committees; AstraZeneca: Other: TRAVEL, ACCOMMODATIONS, EXPENSES. Niesman:MingSight Pharmaceuticals: Current equity holder in private company, Membership on an entity's Board of Directors or advisory committees, Patents & Royalties. Zhang:MingSight Pharmaceuticals: Current Employment, Current equity holder in private company, Membership on an entity's Board of Directors or advisory committees, Patents & Royalties. Woyach:Newave: Consultancy; Loxo: Consultancy; Beigene: Consultancy; AstraZeneca: Consultancy; Abbvie: Consultancy; Schrodinger: Research Funding; Morphosys: Research Funding; Karyopharm: Research Funding; Janssen: Consultancy, Research Funding; Pharmacyclics: Consultancy, Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal